Join A New Vascular Dementia Trial, Coming Soon in 2025
Investigate the Possibility of Improved Memory Following A Stroke.
If you have been diagnosed with Vascular Dementia, you may be eligible for a new clinical trial happening in your area.
Join the Wait List
Hemostemix is planning to recruit patients to participate in the VesCell (ACP-01) Phase I clinical study for Vascular Dementia, which is cognitive decline following a stroke.
The trial will help to evaluate the safety and efficacy of an investigation stem cell therapy (ACP-01) derived from a simple blood draw. Vascular Dementia is a subset of Alzheimer's Disease.
Hemostemix hopes to bring relief to patients and their families through this study.
All information is kept private and follows all HIPAA guidelines.
View the clinical study details at http:www.clinicaltrials.gov
Participation Eligibility
Participant eligibility includes age, gender, type and stage of disease, and previous treatments or health concerns. Guidelines differ from study to study, and identify who can or cannot participate.
There is no guarantee that every individual who qualifies and wants to participate in a trial will be enrolled. Contact the study team to discuss study eligibility and potential participation.
Participating Locations
Study status can change often. Please contact the study team for the most up-to-date information regarding possible participation.
Understanding Vascular Dementia
Learn more about what ViD is, from diagnosis to treatment.
Learn More about Vascular Dementia
Share Your VesCell Story
Share your experience with Vscell to help inform and inspire others. To participate please email support@vescell.health or call 540-878-6754
Your Dedicated Care Team Navigator
Your VesCell Care Team Navigator is committed to getting to know you and your individual needs.
Get to know your Ambassador
.jpg)

Thomas Smeenk
CEO, Hemostemix
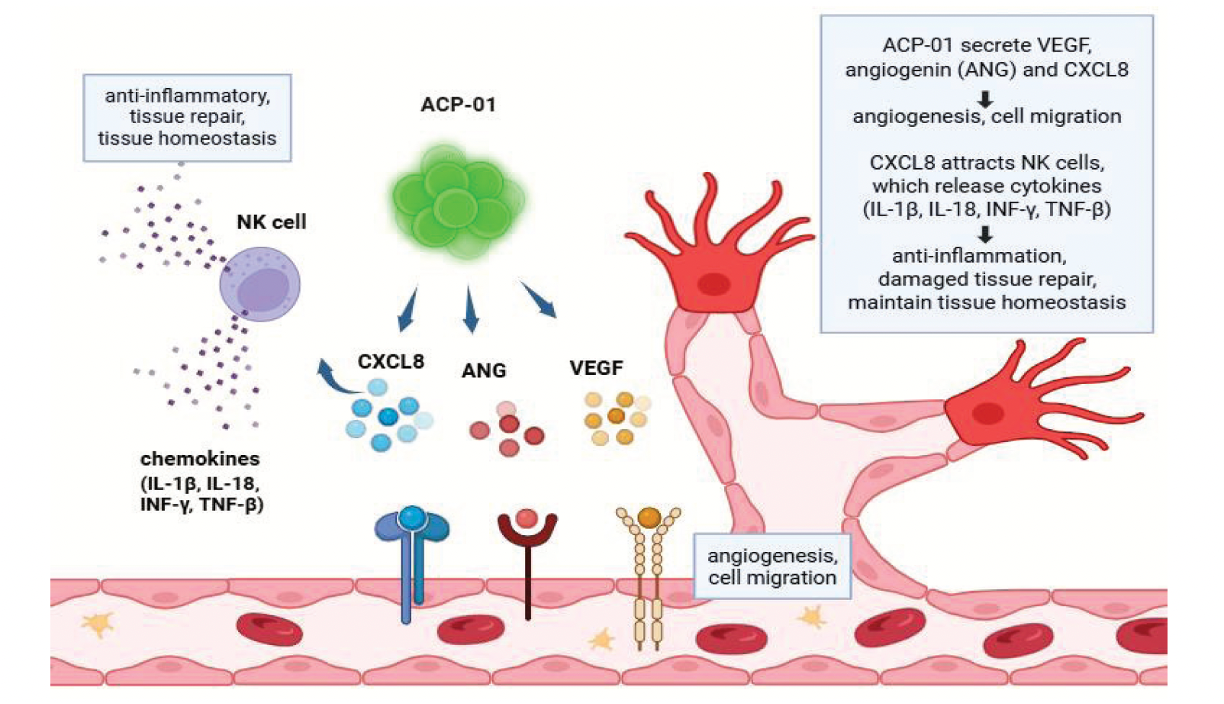
“Listen to a Patient treated with ACP-01 for Vascular Dementia 10 Years Post-Treatment,” stated Thomas Smeenk, CEO, Hemostemix. “Mrs. L lost her daily function-abilities and was to be admitted to a long-term care facility. Ten years after ACP-01 treatment, in 12 minutes, she discussed her career path, family-raising-time, wonderful second marriage, ability to drive, and the benefits of ACP treatment including no longer suffering from ocular migraines. Then she scheduled a lunch appointment with the physician who treated her for vascular dementia, to celebrate her cognitive health ten years post treatment,” Smeenk continued. “With BNA™’s readouts of ACP’s impacts, I predict Hemostemix and Firefly will set the objective standard for the successful diagnoses and treatment of vascular dementia. Families who have a loved-one who are suffering from vascular dementia may contact me (tsmeenk@hemostemix.com), to understand the assessment, inclusion and exclusion criteria,” Smeenk said.
All Vascular Studies
with Autologous Stem Cells (ACP-01)





Important Safety Data
- VesCell (ACP-01) is an investigational therapy under development, not approved by the FDA, and only available in specific countries.
- Treatments are subject to health regulations in Canada, Bahamas, Dominican Republic, and Switzerland, and not US FDA oversite.
- Canada’s Special Access program allows unapproved treatments for serious conditions when conventional therapies have failed.
- U.S. Patients travel at their own risk for treatments received abroad.
- US Patients should consult with their physicians before seeking treatment abroad.
- Treatments are generally out of pocket expenses and may not be covered by US insurance.
Treatment Risk Information
Angiogenic Precursor Cell Treatment of Critical Limb Ischemia Decreases Ulcer Size, Amputation and Death Rate: Re-Examination of phase II ACP NO-CLI Trial Data
Fraser C Henderson*, Ina Sarel, Kelly Tuchman, Stephen Lewis and York Hsiang
Volume5-Issue2
Dates: Received: 2024-01-18 | Accepted: 2024-02-01 | Published: 2024-02-02
Pages: 092-105
Abstract
Introduction: Critical limb ischemia has a prevalence in the US of 1.33%, with mortality 15-20% and major amputation 10-40% per year. Stem cell treatment has emerged as a treatment option for the 45% of patients for whom revascularization procedures are not possible.
Objective: This study re-examines the data of the Phase II clinical treatment of no option Critical limb ischemia with Hemostemix’ angiogenic cell precursors, focusing upon ulcer wound healing, amputation and death rate of this cohort.
Methods: Primary endpoints were changes in ulcer size and major amputation or death within one year of treatment. The secondary endpoint was change in pain level.
Results: From 2015 to 2021, 67 patients with no option Critical limb ischemia were allocated to treatment with ACP-01 (46/67) or placebo (21/67). From this data, only patients who presented with wound ulcers before administration of ACP-01 were reviewed (21 treatment, 8 placebo). Ulcer size in the treated group decreased from a mean of 1.46 cm2 to 0.48 mm2 (p = 0.01) by 3 months. There was no significant decrease in the size of the ulcers of the placebo group (p < 0.54). At one year there were no complications related to treatment. The treatment group had one amputation (4.8%) and one death (4.8%); the placebo group had 2 amputations (25%) and 1 death (12.5%). Change in pain was not significant in either group at 3 months, but at 1 year was improved in the placebo group (p = 0.01).
Conclusion: The administration of ACP-01 within a program of careful patient follow up is safe and associated with reduced ulcer size and decreased rate of amputation and death. Consideration should be given to re-administration of stem cell treatments every 3-6 months to optimize improvement of Critical limb ischemia. Further studies, more appropriately powered, are warranted.