Society spent a generation focused on managing end stage disease
But what does the science say?
We can give our cells a fighting chance.
As Peripheral Arterial Disease (PAD) is characterized by identifiable symptoms.
Cold, pale, or bluish feet, weak or absent pulse in the legs, muscle atrophy.
Today's standard of care.
Including re-vascularization, surgical interventions, anti-coagulation, wound care, and pain management.
- Based on guidelines from the Society for Vascular Surgery (SVS), American College of Cardiology (ACC), and European Society of Cardiology (ESC), the priority is timely revascularization, with endovascular approaches often preferred initially due to lower morbidity
- Requires a disciplinary approach involving vascular surgeons, interventional radiologists, wound care specialists, podiatrists, and physical therapists.
- Goal is to preserve as much functional limb as possible (e.g., below-knee vs. above-knee amputation).
- Amputation considered when revascularization fails, is not feasible, or in cases of severe infection, extensive tissue loss, or non-functional limb.
The Cost of Care is High.
The cost for a PAD patient is approximately $150,000 can exceed $300,000, particularly if patients need multiple hospitalizations, amputations, or extensive wound care.
Evidence suggests ACP-01 may improve circulation.
ACP-01 may have a distinct advantage - by targeting several underlying disease mechanisms at the same time. This may decrease limb amputation in 4 ways.
93.5% of limbs treated with ACP-01 in a randomized double blind phase II clinical trial experienced healing of ulcers and cessation of pain (1).
New Blood Vessel Formation
ACP-01 is:
(1) Programmed to form blood vessels.
(2) Exerts a potent paracrine effect, by secreting vascular endothelial growth factor (VEGF) and angiogenin.
(3) Is enriched with CD34+ cells, which amplify the angiogenic response to improve blood flow through the leg.
Cell Migration to Areas of Decreased Blood Flow
ACP-01 expresses surface receptors (CXCR4), which are attracted to proteins (chemokine CXCL12) released by the ischemic leg tissue. The ACP-01 cells embed and repopulate injured tissue, releasing growth factors to promote healing.
Healing and Regeneration of Blood Vessels
Release Interleukin 8 which attracts CD 34+ cells from the peripheral circulation to improve circulation.
(1) Decreases cell death (anti-apoptotic) by increasing the expression of molecules such as the Nuclear Factor Kappa B.
(2) Improve healing and regeneration of blood vessels and injured tissue by attracting cells (macrophages) that clear cell debris but minimize inflammation.
(3) ACP-01 promotes “M2 Anti-inflammatory” healing.
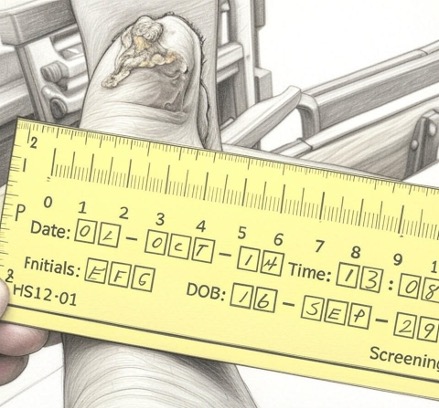
Before
ACP-01 Treatment
Dry wound involving the distal tip of D1 immediately prior to treatment.
Four months post treatment showing significant improvement. Concurrent treatment wearing an offloading orthotic and regular dressing changes.
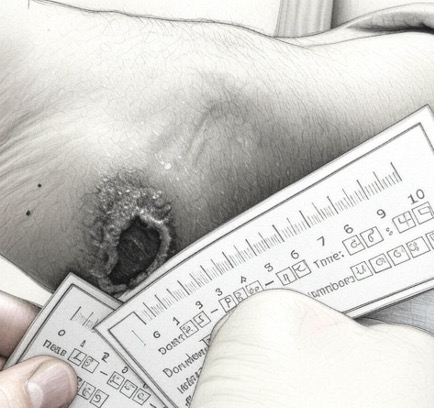
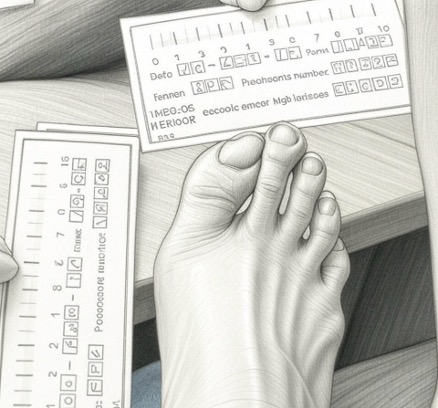
After
ACP-01 Treatment
The wound healed four weeks after treatment with no evidence of recurrence.
Four months post treatment showing significant improvement. Concurrent treatment wearing an offloading orthotic and regular dressing changes.
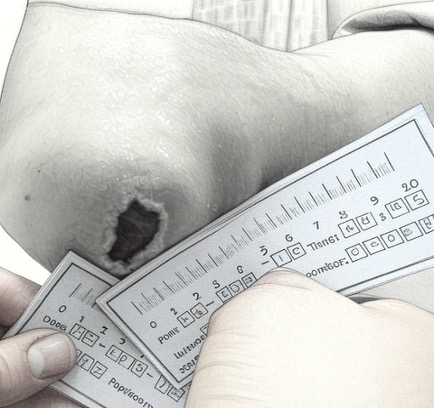
A New Path for No-Option Critical Limb Ischemia
For those facing critical limb ischemia (CLI) with no other treatment options, VesCell offers hope.
This investigational autologous stem cell therapy is designed to improve blood flow in the lower legs and feet, heal ulcers, and reduce the risk of amputation and mortality.
Critical limb ischemia (CLI) is a subset of Peripheral Artery Disease (PAD).
Backed by Phase II clinical trial data, VesCell is an investigation treatment for patients with severe CLI.
Realizing our full potential
As we age, the burden of cardiovascular and cognitive disease will increase pressure on healthcare resources. Therapies that can improve quality of life, reduce hospitalization, delay surgeries, and diminish the requirement for long-term care offer systemic sustainability in addition to clinical benefit. Stem Cells are positioned to play a central role in this landscape.





Important Safety Data
- VesCell (ACP-01) is an investigational therapy under development, not approved by the FDA, and only available in specific countries.
- Treatments are subject to health regulations in Canada, Bahamas, Dominican Republic, and Switzerland, and not US FDA oversite.
- Canada’s Special Access program allows unapproved treatments for serious conditions when conventional therapies have failed.
- U.S. Patients travel at their own risk for treatments received abroad.
- US Patients should consult with their physicians before seeking treatment abroad.
- Treatments are generally out of pocket expenses and may not be covered by US insurance.
Treatment Risk Information
Angiogenic Precursor Cell Treatment of Critical Limb Ischemia Decreases Ulcer Size, Amputation and Death Rate: Re-Examination of phase II ACP NO-CLI Trial Data
Fraser C Henderson*, Ina Sarel, Kelly Tuchman, Stephen Lewis and York Hsiang
Volume5-Issue2
Dates: Received: 2024-01-18 | Accepted: 2024-02-01 | Published: 2024-02-02
Pages: 092-105
Abstract
Introduction: Critical limb ischemia has a prevalence in the US of 1.33%, with mortality 15-20% and major amputation 10-40% per year. Stem cell treatment has emerged as a treatment option for the 45% of patients for whom revascularization procedures are not possible.
Objective: This study re-examines the data of the Phase II clinical treatment of no option Critical limb ischemia with Hemostemix’ angiogenic cell precursors, focusing upon ulcer wound healing, amputation and death rate of this cohort.
Methods: Primary endpoints were changes in ulcer size and major amputation or death within one year of treatment. The secondary endpoint was change in pain level.
Results: From 2015 to 2021, 67 patients with no option Critical limb ischemia were allocated to treatment with ACP-01 (46/67) or placebo (21/67). From this data, only patients who presented with wound ulcers before administration of ACP-01 were reviewed (21 treatment, 8 placebo). Ulcer size in the treated group decreased from a mean of 1.46 cm2 to 0.48 mm2 (p = 0.01) by 3 months. There was no significant decrease in the size of the ulcers of the placebo group (p < 0.54). At one year there were no complications related to treatment. The treatment group had one amputation (4.8%) and one death (4.8%); the placebo group had 2 amputations (25%) and 1 death (12.5%). Change in pain was not significant in either group at 3 months, but at 1 year was improved in the placebo group (p = 0.01).
Conclusion: The administration of ACP-01 within a program of careful patient follow up is safe and associated with reduced ulcer size and decreased rate of amputation and death. Consideration should be given to re-administration of stem cell treatments every 3-6 months to optimize improvement of Critical limb ischemia. Further studies, more appropriately powered, are warranted.

